Tability is a cheatcode for goal-driven teams. Set perfect OKRs with AI, stay focused on the work that matters.
What are Regulatory Affairs Team OKRs?
The OKR acronym stands for Objectives and Key Results. It's a goal-setting framework that was introduced at Intel by Andy Grove in the 70s, and it became popular after John Doerr introduced it to Google in the 90s. OKRs helps teams has a shared language to set ambitious goals and track progress towards them.
Formulating strong OKRs can be a complex endeavor, particularly for first-timers. Prioritizing outcomes over projects is crucial when developing your plans.
We've tailored a list of OKRs examples for Regulatory Affairs Team to help you. You can look at any of the templates below to get some inspiration for your own goals.
If you want to learn more about the framework, you can read our OKR guide online.
The best tools for writing perfect Regulatory Affairs Team OKRs
Here are 2 tools that can help you draft your OKRs in no time.
Tability AI: to generate OKRs based on a prompt
Tability AI allows you to describe your goals in a prompt, and generate a fully editable OKR template in seconds.
- 1. Create a Tability account
- 2. Click on the Generate goals using AI
- 3. Describe your goals in a prompt
- 4. Get your fully editable OKR template
- 5. Publish to start tracking progress and get automated OKR dashboards
Watch the video below to see it in action 👇
Tability Feedback: to improve existing OKRs
You can use Tability's AI feedback to improve your OKRs if you already have existing goals.
- 1. Create your Tability account
- 2. Add your existing OKRs (you can import them from a spreadsheet)
- 3. Click on Generate analysis
- 4. Review the suggestions and decide to accept or dismiss them
- 5. Publish to start tracking progress and get automated OKR dashboards
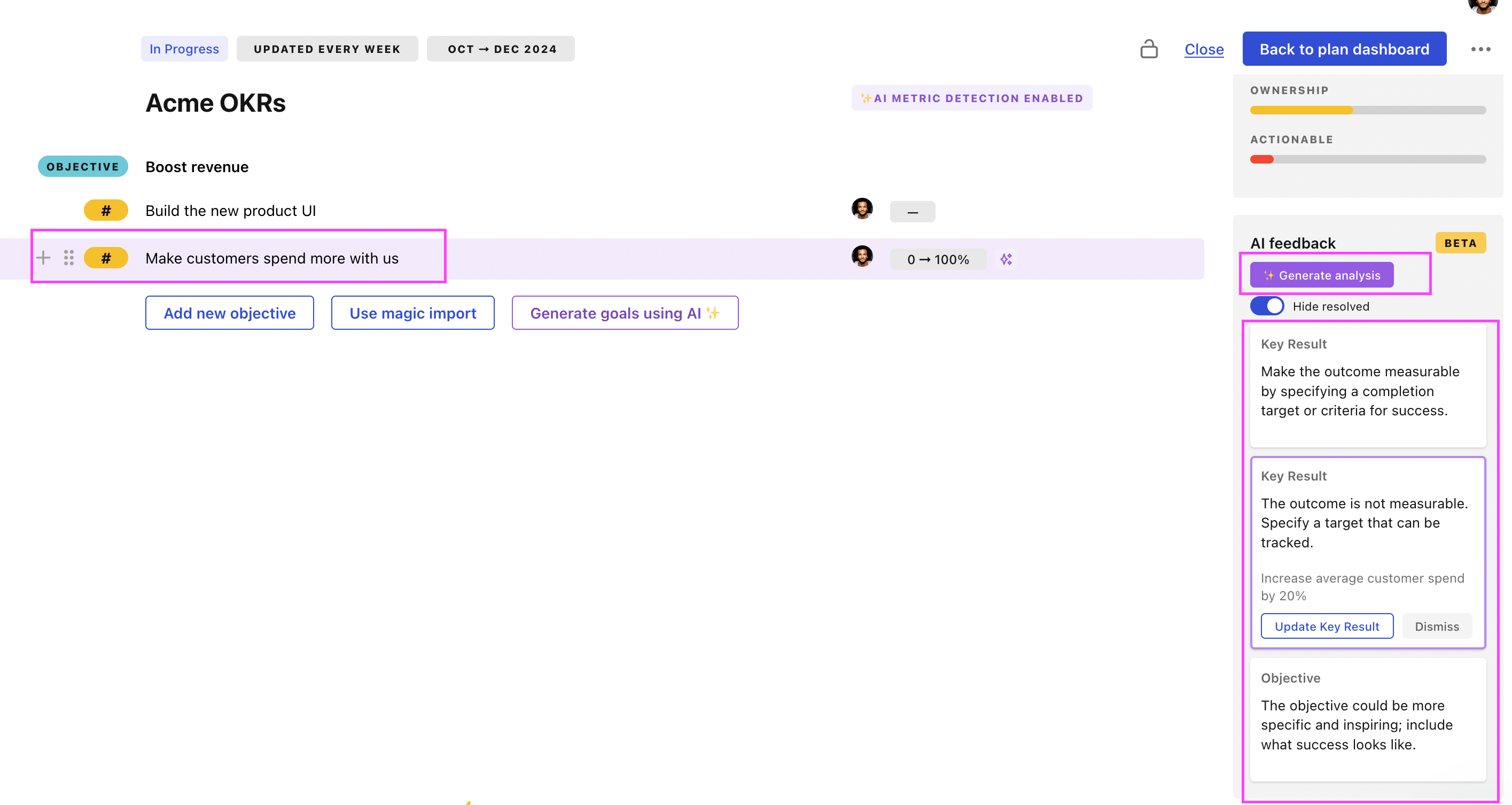
Tability will scan your OKRs and offer different suggestions to improve them. This can range from a small rewrite of a statement to make it clearer to a complete rewrite of the entire OKR.
Regulatory Affairs Team OKRs examples
You'll find below a list of Objectives and Key Results templates for Regulatory Affairs Team. We also included strategic projects for each template to make it easier to understand the difference between key results and projects.
Hope you'll find this helpful!
OKRs to develop innovative pharmaceutical products
ObjectiveDevelop innovative pharmaceutical products
KRDevelop and test 2 prototypes with satisfactory patient outcomes
Conduct patient outcome tests on prototypes
Identify key components for prototype based on patient needs
Build and refine two prototype designs
KRConduct research to identify 3 potential active pharmaceutical ingredients
Identify key drugs suitable for pharmaceutical component research
Conduct thorough research on potential active ingredients
Document findings for each potential ingredient
KRSecure regulatory approval for 1 new pharmaceutical product
Respond promptly to regulatory feedback and additional information requests
Conduct rigorous clinical trials for safety and efficacy
Develop and submit detailed dossier to regulatory authority
OKRs to secure FDA approval for our new pharmaceutical product
ObjectiveSecure FDA approval for our new pharmaceutical product
KRResolve all FDA queries or issues regarding the application within six weeks
Research and compile thorough responses to each issue
Submit all responses and corrections to FDA within six weeks
Identify all FDA queries or issues on the application
KRSubmit a complete and compliant application to FDA within the first month
Review FDA guidelines to ensure application compliance
Submit the completed application to the FDA
Gather all necessary documents and data for application
KRSuccessfully pass the FDA's inspection and audit of our production facilities
Ensure all documentation and records are accurate, updated, and easily accessible
Provide thorough training to staff on FDA regulations and requirements
Maintain the facility's cleanliness and safety according to FDA standards
OKRs to secure FDA approval for the capital equipment medical device
ObjectiveSecure FDA approval for the capital equipment medical device
KRComplete and submit comprehensive FDA application by week 4
Thoroughly fill out the FDA application form accurately and comprehensively
Gather all necessary documentation and information for FDA application
Submit the completed FDA application before the deadline in week 4
KRObtain confirmation of FDA approval by end of the quarter
Regularly follow-up with FDA regarding application status
Prepare and submit all necessary documentation for FDA approval
Ensure all response deadlines to FDA inquiries are met
KRPass all necessary FDA inspections successfully with zero citations
Implement strict internal quality control measures
Regularly inspect and document all processes for compliance
Review and comprehend all FDA guidelines and expectations
OKRs to Improve the efficiency of our corporate affairs operations
Objective Improve the efficiency of our corporate affairs operations
KRImplement new communication strategies, improving internal communication efficiency by 25%
Monitor and analyze communication improvements
Research and choose a suitable internal communication system
Train employees on utilizing the new communication system
KRFacilitate at least 2 training programs to improve staff's understanding of corporate policies
Identify necessary areas for training in current corporate policies
Develop comprehensive training programs tailored to these areas
Schedule and conduct two policy training sessions
KRAchieve a 30% reduction in time taken for regulatory compliance processes
Streamline documentation processes for increased efficiency
Implement automation software for routine compliance tasks
Train staff in fast, effective compliance procedures
OKRs to enhance the quality and regulatory compliance of debt collection practices
ObjectiveEnhance the quality and regulatory compliance of debt collection practices
KRComplete 100% of mandatory compliance trainings for all team members
Monitor and track team members' training progress
Set deadlines for completing each training course
Identify all mandatory compliance trainings for each team member
KRImplement a 15% improvement in quality assurance scores from customer feedback
Analyze customer feedback and identify areas needing improvement
Train staff on identified areas to rectify issues
Implement customer-directed quality assurance initiatives
KRReduce non-compliance issues by 20% through periodic audits and refinements
Establish process for identifying and correcting non-compliance
Implement follow-up reviews to confirm resolutions
Develop a schedule for regular compliance audits
Regulatory Affairs Team OKR best practices
Generally speaking, your objectives should be ambitious yet achievable, and your key results should be measurable and time-bound (using the SMART framework can be helpful). It is also recommended to list strategic initiatives under your key results, as it'll help you avoid the common mistake of listing projects in your KRs.
Here are a couple of best practices extracted from our OKR implementation guide 👇
Tip #1: Limit the number of key results
Having too many OKRs is the #1 mistake that teams make when adopting the framework. The problem with tracking too many competing goals is that it will be hard for your team to know what really matters.
We recommend having 3-4 objectives, and 3-4 key results per objective. A platform like Tability can run audits on your data to help you identify the plans that have too many goals.
Tip #2: Commit to weekly OKR check-ins
Setting good goals can be challenging, but without regular check-ins, your team will struggle to make progress. We recommend that you track your OKRs weekly to get the full benefits from the framework.
Being able to see trends for your key results will also keep yourself honest.
Tip #3: No more than 2 yellow statuses in a row
Yes, this is another tip for goal-tracking instead of goal-setting (but you'll get plenty of OKR examples above). But, once you have your goals defined, it will be your ability to keep the right sense of urgency that will make the difference.
As a rule of thumb, it's best to avoid having more than 2 yellow/at risk statuses in a row.
Make a call on the 3rd update. You should be either back on track, or off track. This sounds harsh but it's the best way to signal risks early enough to fix things.
Save hours with automated Regulatory Affairs Team OKR dashboards
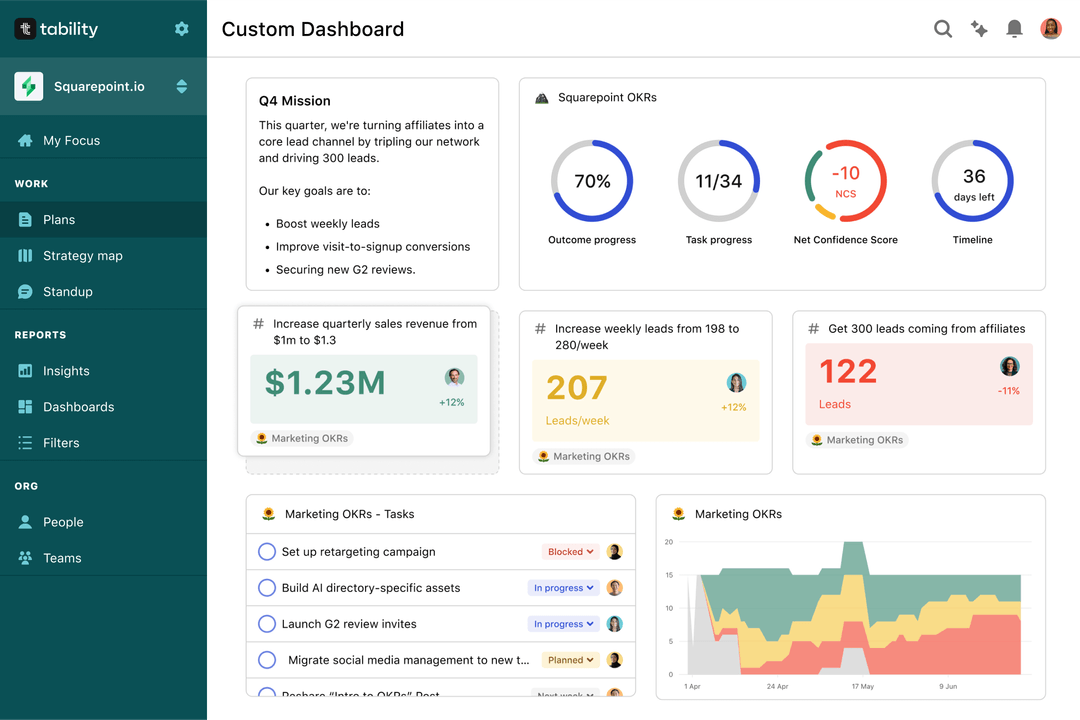
Your quarterly OKRs should be tracked weekly if you want to get all the benefits of the OKRs framework. Reviewing progress periodically has several advantages:
- It brings the goals back to the top of the mind
- It will highlight poorly set OKRs
- It will surface execution risks
- It improves transparency and accountability
Spreadsheets are enough to get started. Then, once you need to scale you can use Tability to save time with automated OKR dashboards, data connectors, and actionable insights.
How to get Tability dashboards:
- 1. Create a Tability account
- 2. Use the importers to add your OKRs (works with any spreadsheet or doc)
- 3. Publish your OKR plan
That's it! Tability will instantly get access to 10+ dashboards to monitor progress, visualise trends, and identify risks early.
More Regulatory Affairs Team OKR templates
We have more templates to help you draft your team goals and OKRs.
OKRs to streamline marketing campaign processes for improved efficiency and consistency
OKRs to implement a science mentoring program for skill enhancement
OKRs to enhance efficacy of fraud detection/rules mechanism to minimize customer impact
OKRs to enhance customer service and satisfaction in schools
OKRs to boost overall sales productivity
OKRs to be on track for a long-running project